Caco3 Solubility in Water
We can consult a solubility table to check and determine if CaCO3 will dissolve in water. The estimated solubility would be 71x10-5M.

Is Caco3 Soluble Or Insoluble In Water Youtube
There are also a set of general rules to help remember which compounds are soluble insoluble or.

. CaCO3 has very low solubility in water. The fact is calcium carbonate is insoluble and calcium bicarbonate is soluble in water. A lot of carbonates like aragonite CaMgCO 3 have a pKa of about 83 which is also sea water pH.
Calcium carbonate has a very low solubility in pure water 15 mgL at 25C but in rainwater saturated with carbon dioxide its solubility increases due to the formation of more soluble. Carbonic Acid Calcium Salt. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressure.
In aqueous solution such salts undergo hydrolysis to give free OH-. According to wikipedia C a H C O X 3 X 2 has the following solubility values. It is a salt of a fairly strong base calcium hydroxide and a weak acid carbonic acid.
It is a salt of a fairly strong base calcium hydroxide and a weak acid carbonic acid. They will dissolve at an increasingly slower rate as they approach that. Because it is insoluble in water we would expect that it would NOT dissociate into its ions Ca 2 and CO3 2-.
HCO 3 - H CO 3 2-K 2 H CO 3 2- HCO 3. The solubility or calcium carbonate in pure water is 69 x 10-5 moles per liter. What is the concentration of calcium ions in a.
Released carbonate ions from CaCO3 dissolution directly influence the reaction equilibrium between CO2 and water and therefore the change in pH value of the solution. 161 g 100 m L 0 C 166 g 100 m L 20 C 184 g 100 m L 100 C So I assume C a H C. 1Ca2 2HCO3 CaCO3s CO2 H2O Thus as pressure decreases CO 2 is.
What is the solubility of CaCO3 in pure water. This immediately will lead us to conclude that hydration energy lattice energy in. Firstly the precipitation of CaCO 3 is controlled by the following equilibrium 1.
On the solubility table we can see that CaCO3 is insoluble in water. The equilibrium solubility of four calcium salts calcium oxalate hydrate calcium citrate tetrahydrate calcium phosphate calcium glycerophosphate were determined at controlled pH. In aqueous solution such salts undergo hydrolysis.
Find Supelco-CX0120 MSDS related peer-reviewed papers technical. We need to take into account the hydrolysis of the carbonate ion with K 2 48x10-11. CaCO3 has very low solubility in water.

Solubility Curve Of Different Calcium Carbonate Forms Warsinger Et Download Scientific Diagram
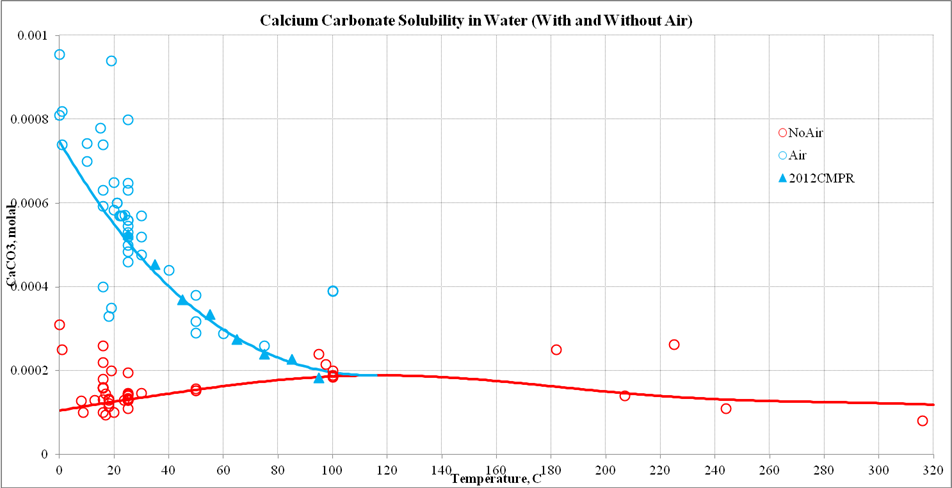
Calcium Carbonate Solubility Wiki Olisystems Com

Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram
Chemistry Lesson Calcium Carbonate Solubility Lucky Sci

Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram

Comments
Post a Comment